5 things to know about the Pfizer vaccine

Pfizer Inc. said Monday that its COVID-19 vaccine may be a remarkable 90% effective, based on early and incomplete test results.
It brought a big burst of optimism to a world desperate for the means to finally bring the catastrophic outbreak under control.
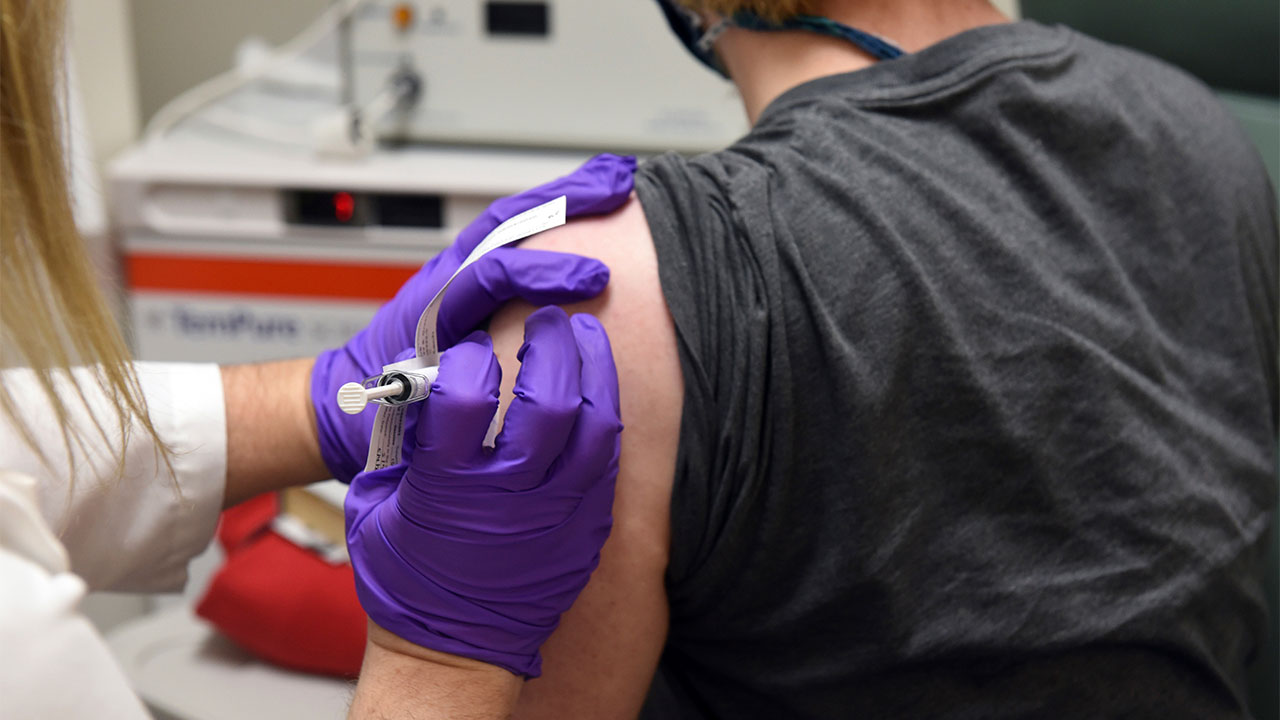
Here are five things to know about the Pfizer vaccine:
You will have to wait to get it
Pfizer, which is developing the vaccine with its German partner BioNTech, now is on track to apply later this month for emergency-use approval from the U.S. Food and Drug Administration, once it has the necessary safety information in hand.
Even if all goes well, authorities have stressed it is unlikely any vaccine will arrive much before the end of the year, and the limited initial supplies will be rationed.
The results are "extraordinary," according to officials.
Dr. Anthony Fauci, the U.S. government's top infectious-disease expert, said the results suggesting 90% effectiveness are "just extraordinary," adding: "Not very many people expected it would be as high as that."
"It's going to have a major impact on everything we do with respect to [COVID-19,]" Fauci said as Pfizer appeared to take the lead in the all-out global race by pharmaceutical companies and various countries to develop a well-tested vaccine against the virus.
Dr. Bruce Aylward, the World Health Organization's senior adviser, said Pfizer's vaccine could "fundamentally change the direction of this crisis" by March, when the U.N. agency hopes to start vaccinating high-risk groups.
Pfizer CEO says 3rd week of November earliest it can seek COVID-19 vaccine OK

Pfizer's vaccine is among four candidates already in huge studies in the U.S.
Another U.S. company, Moderna Inc., also hopes to file an application with the FDA late this month.
Both companies' shots are made with a brand-new technology. These "mRNA vaccines" aren't made with the coronavirus itself, meaning there's no chance anyone could catch it from the shots. Instead, the vaccine contains a piece of genetic code that trains the immune system to recognize the spiked protein on the surface of the virus.
Don't forget safety
Safety is the top priority. Monitors watch for unexpected or serious side effects. Earlier this fall, separate studies of vaccine candidates made by AstraZeneca and Johnson & Johnson were temporarily halted after some participants experienced health problems, delaying the research until safety investigations allowed both to resume.
Pfizer said Monday no serious safety concerns have emerged so far with its vaccine.
But the FDA is requiring that companies track at least half of the study volunteers for two months to look for side effects before asking the agency to review their vaccine. That's about when side effects have cropped in studies of other vaccines.
Pfizer and Moderna both expect to reach that safety milestone later in November.
Here's what the COVID-19 vaccine rollout could look like in the U.S.

What happens then?
Companies are expected to seek permission for "emergency use" of their vaccines, rather than waiting to fully complete their studies and then seeking traditional approval.
The FDA's scientific advisers will debate each company's study findings in a public meeting before the agency decides.
Manufacturers already have begun stockpiling vaccine doses in anticipation of eventual approval, but the first shots will be in short supply and rationed. And the first people vaccinated will need to undergo extra safety tracking, as the government watches for rare side effects that might crop up when the shots are given to many more people than were in the research studies.
Here's what it'll take to get a COVID-19 vaccine and how it'll be made










